Change of State
Change of State: Overview
This Topic covers sub-topics such as Latent Heat, Deposition, Melting Point of Substances, Temperature Versus Heat Graph for Heating of Ice, Vaporisation of Substances and, Fusion of Substances
Important Questions on Change of State
An ice cube is heated and the variation of its temperature with time is shown. The process representing the conversion of water into steam is
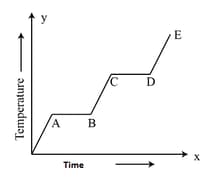
When an ice cube is heated to vapour, in which all situations, will it have two states at the same temperature? Explain.
An ice cube is kept on the gas stove in a tumbler. It is heated with a gas. What will happen to it after some time? Explain the change of states associated with this.
Evaporation is the process of changing liquid into vapor,
What is the process of conversion of substances from their vapour state to a solid state upon cooling called?
A block of ice at is slowly heated and converted to steam at . Which of the following curves represent the phenomenon qualitatively?
The process in which a solid is converted to gaseous state is called _____.
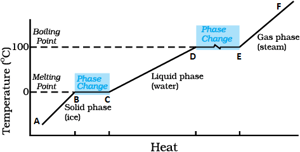
Identify the part representing latent heat of vaporization in the temperature vs heat graph for melting of ice.
Liquid and solid states of a substance co-exist in thermal equilibrium during the process of:
Which material has a high latent heat of fusion particular to its composition?
A block of ice at is slowly heated and converted to steam at . Which of the following curves represent the phenomenon qualitatively:
